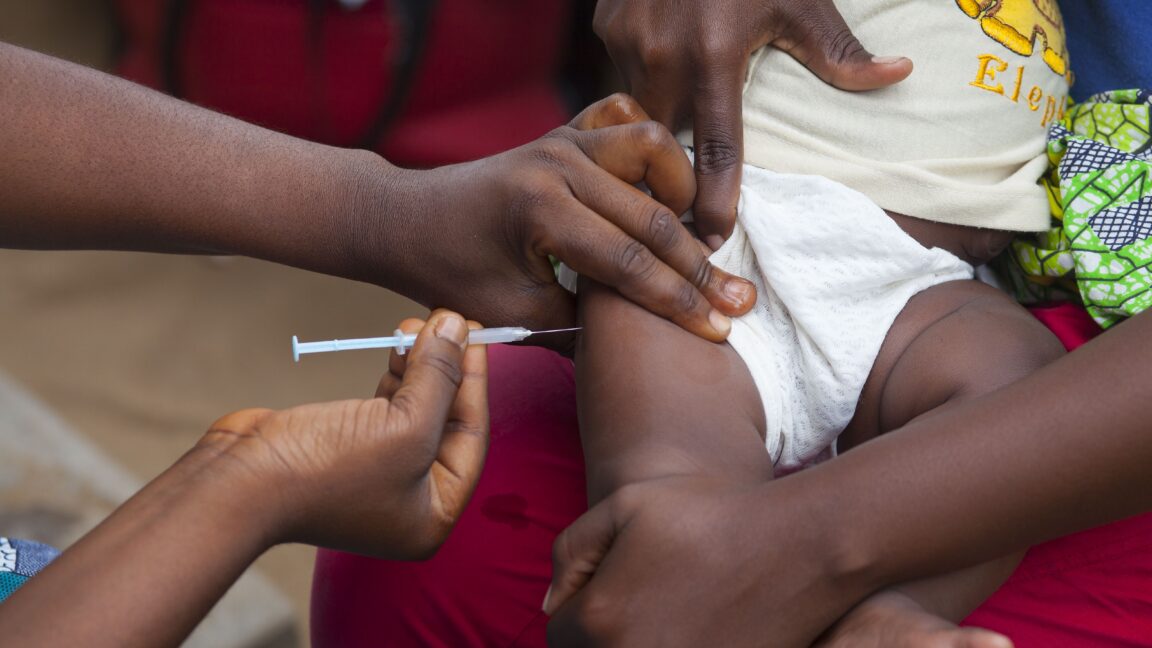
The World Health Organization has formally declared a US-funded hepatitis B vaccine trial in Guinea-Bissau as “unethical” because it withholds a safe and potentially life-saving vaccine from some newborns. This condemnation stems from the trial’s design, which the WHO states is inconsistent with established ethical and scientific principles. The US Centers for Disease Control and Prevention awarded $1.6 million to Danish researchers for the trial, which has been met with widespread criticism from health experts for its methodology and the researchers’ past controversial practices. The trial’s proposed randomization of newborns to receive the hepatitis B vaccine at birth or at six weeks, withholding the birth dose for some, is particularly concerning given Guinea-Bissau’s planned transition to a birth dose recommendation.
Read the original article here
The World Health Organization has voiced strong disapproval, characterizing a US-funded newborn vaccine trial as “unethical.” This condemnation stems from serious concerns about the trial’s design, its potential implications for public health, and the exploitation of vulnerable populations. At the heart of the controversy is a study planned to administer the Hepatitis B vaccine to 14,000 infants in Guinea-Bissau, with the intervention group receiving it at birth and the control group at six weeks.
The fundamental issue raised by the WHO is the unnecessary withholding of a proven, life-saving intervention. The Hepatitis B vaccine has been in widespread use for three decades across 115 countries, establishing a robust track record of safety and efficacy. To deliberately delay its administration to a portion of newborns, especially in a region where Hepatitis B is prevalent, is seen as exposing infants to significant and preventable risks. These risks include chronic infection, cirrhosis, and potentially liver cancer later in life, outcomes that are entirely avoidable with timely vaccination.
Furthermore, the context in which this trial is proposed is deeply problematic. Guinea-Bissau is already planning to transition to routine birth dose Hepatitis B vaccination by 2028. However, resource constraints currently prevent them from implementing this sooner. The WHO views the trial as exploiting this existing scarcity. Instead of directing resources towards supporting Guinea-Bissau’s efforts to achieve timely universal vaccination, the trial appears to leverage the country’s limitations for experimental purposes, raising questions about the true beneficiaries and motivations behind the study.
The prevalence of Hepatitis B in Guinea-Bissau is a significant factor amplifying the ethical concerns. Reports suggest that as many as 12% of adults in the country have Hepatitis B, and some medical professionals working with migrant communities from Guinea-Bissau indicate that this figure may even be an underestimation. This high disease burden underscores the urgency of timely vaccination and the inherent danger of delaying the Hepatitis B vaccine for newborns in such an environment.
Beyond the immediate risk of infection, the methodology of the trial itself has drawn sharp criticism. Concerns have been raised about the trial’s design being less rigorous and more open to interpretation than established research standards. The implication is that this could subtly influence the results, leading to a predetermined conclusion rather than an objective assessment of the vaccine’s safety. Specifically, the trial’s design is described as single-blind and lacking a true placebo control, which significantly increases the risk of bias and limits the reliability and policy relevance of any findings.
Compounding these ethical quandaries is the apparent discrepancy between the intentions of the research and the stance of the host country. Reports indicate that Guinea-Bissau’s government has expressed concerns about the trial’s ethical implications and has indicated it should not proceed. However, the Centers for Disease Control and Prevention (CDC), a key funder of the trial, has asserted that it will move forward, creating a conflict between national sovereignty and the decisions of external funding bodies. Conducting an experiment on the citizens of a sovereign nation without their full and uncoerced agreement is a serious breach of international ethical norms.
The rationale behind conducting such a study, particularly when the vaccine is already well-established and its benefits are widely accepted, is difficult to reconcile with established scientific and ethical principles. The argument that a proven intervention should be withheld from some participants to investigate alleged safety concerns, for which there is no credible supporting evidence, is seen as fundamentally flawed. Critics point out that the potential for harm far outweighs any speculative benefits of such a trial, especially when alternative research methods, such as using existing data from populations that have not received the vaccine, could be employed.
The involvement of a controversial research organization with a history of questionable ethics and methods in vaccine safety debates further fuels the controversy. This background, coupled with the decision by the US to fund a study in Africa rather than within its own borders, raises questions about equity and the ethical treatment of populations in lower-income settings. The perception is that vulnerable populations are being exploited for research purposes, echoing past instances of unethical human experimentation.
In essence, the WHO’s strong stance reflects a deep concern that this trial represents a disregard for established public health principles, jeopardizes infant health, and undermines the trust in vaccination programs globally. The criticism highlights the critical importance of ethical oversight, scientific rigor, and respect for national sovereignty when conducting research involving human subjects, especially in vulnerable populations. The trial’s suspension pending further review suggests that these ethical concerns are being taken seriously, but the WHO’s pronouncement underscores the significant hurdles the study faces in meeting fundamental ethical standards.